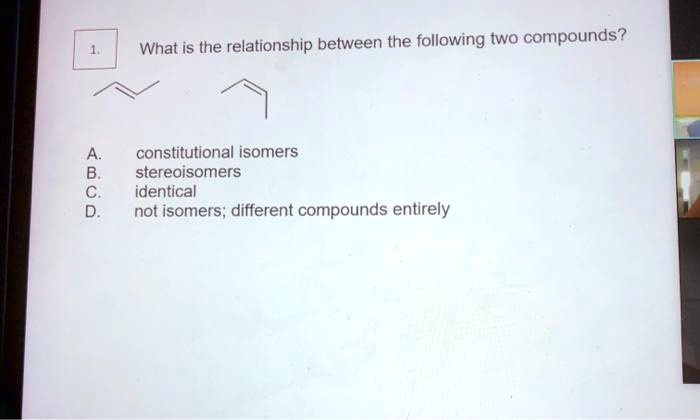
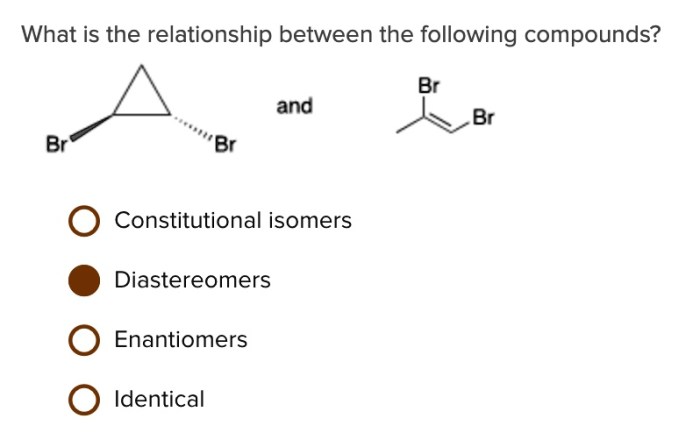
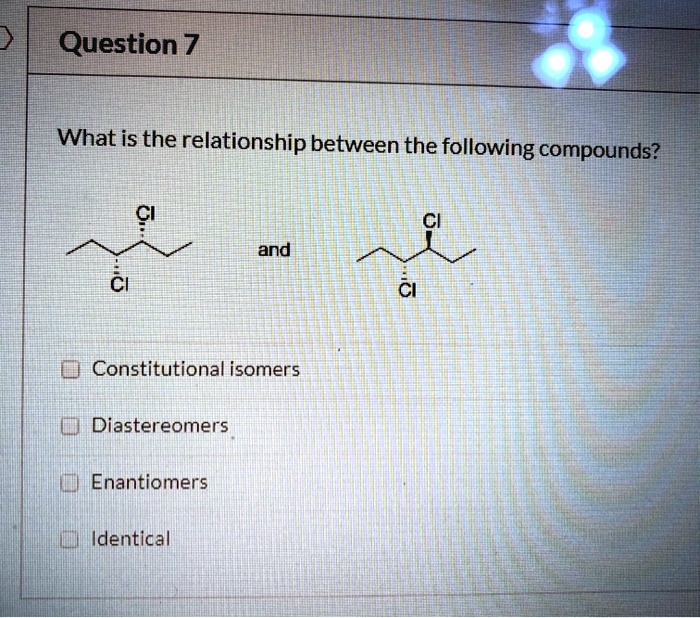
In the realm of chemistry, constitutional isomers stand out as compounds with the same molecular formula yet distinct structural arrangements. Which of the following compounds are constitutional isomers? Embark on a journey to unravel the intricacies of molecular structure and its profound impact on compound properties and reactivity.
Constitutional isomers arise from variations in the connectivity of atoms within a molecule. Their unique structural fingerprints endow them with distinct physical and chemical characteristics, shaping their applications in diverse fields.
Definition of Constitutional Isomers
Constitutional isomers are compounds with the same molecular formula but different structural formulas. This means that they have the same number and type of atoms, but the atoms are arranged in a different order. For example, butane and isobutane are constitutional isomers.
They both have the molecular formula C4H10, but butane has a straight chain of four carbon atoms, while isobutane has a branched chain of three carbon atoms.
Methods for Identifying Constitutional Isomers

There are several methods that can be used to identify constitutional isomers. One method is to count the number of carbon atoms and other atoms in the molecule. For example, butane and isobutane have the same molecular formula, C4H10, but they have a different number of carbon atoms.
Butane has four carbon atoms, while isobutane has three carbon atoms.
Examples of Constitutional Isomers
There are many examples of constitutional isomers. Some common examples include:
- Butane and isobutane
- Pentane and isopentane
- Hexane and isohexane
- Heptane and isoheptane
- Octane and isooctane
Properties and Reactivity of Constitutional Isomers: Which Of The Following Compounds Are Constitutional Isomers
Constitutional isomers have similar physical and chemical properties. However, there are some differences in their reactivity. For example, butane is more reactive than isobutane. This is because the branched chain of isobutane makes it more difficult for the molecule to react with other molecules.
Applications of Constitutional Isomerism
Constitutional isomerism has many applications in chemistry. For example, constitutional isomers are used in the design of drugs and materials. They are also used in organic synthesis.
Top FAQs
What are constitutional isomers?
Constitutional isomers are compounds with the same molecular formula but different structural formulas, resulting from variations in the connectivity of atoms.
How can constitutional isomers be identified?
Constitutional isomers can be identified by analyzing the connectivity of atoms within their structural formulas or using systematic nomenclature.
What are the applications of constitutional isomerism?
Constitutional isomerism finds applications in drug design, material science, and organic synthesis, among other fields.