Biochemistry functional groups the scariest worksheet ever – Embark on a captivating exploration of biochemistry functional groups, the building blocks of life, through the lens of the infamous “scariest worksheet ever.” This comprehensive guide unveils the secrets of these molecular players, their diverse roles, and their significance in shaping the world of biochemistry.
Delve into the fascinating realm of functional groups, the atomic arrangements that define the reactivity and behavior of molecules. Discover their intricate classification, their contributions to the structure and function of biological molecules, and their profound impact on chemical reactions and metabolic pathways.
1. Introduction
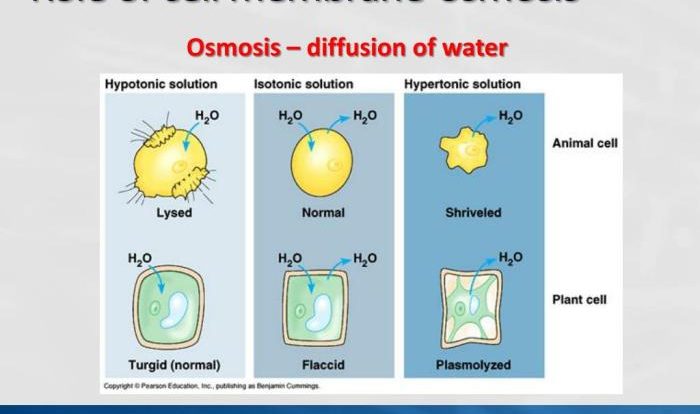
Biochemistry is the study of the chemical processes that occur within living organisms. Functional groups are specific arrangements of atoms within a molecule that determine its chemical properties. They play a crucial role in biochemistry, influencing the reactivity, solubility, and interactions of molecules.
2. Classification of Functional Groups
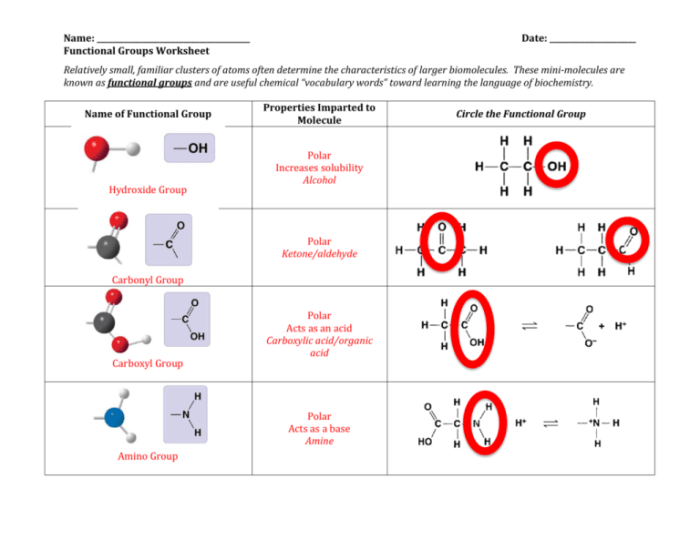
2.1. Hydroxyl Group (-OH)
- Polar and hydrophilic
- Forms hydrogen bonds
- Found in alcohols, carbohydrates, and amino acids
2.2. Carbonyl Group (C=O)
- Polar and electrophilic
- Reacts with nucleophiles
- Found in aldehydes, ketones, and carboxylic acids
2.3. Amino Group (-NH2), Biochemistry functional groups the scariest worksheet ever
- Polar and basic
- Protonates at physiological pH
- Found in amino acids and proteins
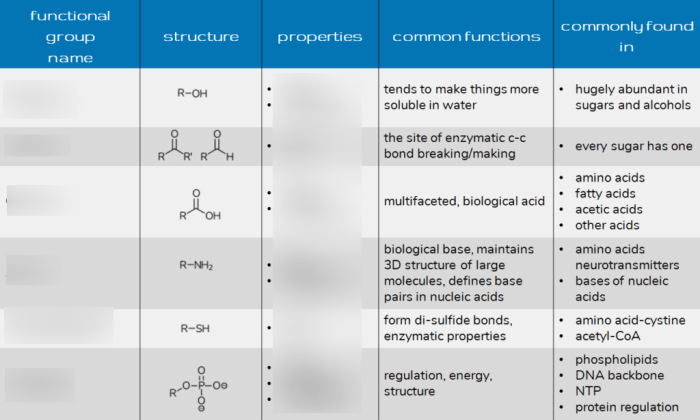
3. Functional Groups and Biological Molecules
Functional groups contribute to the structure and function of biological molecules:
3.1. Carbohydrates
- Contain hydroxyl groups for solubility
- Glycosidic bonds between sugars form via hydroxyl groups
3.2. Proteins
- Amino groups form peptide bonds
- Carboxyl groups provide acidic properties
- Hydroxyl groups contribute to protein folding
4. Functional Groups and Chemical Reactions: Biochemistry Functional Groups The Scariest Worksheet Ever
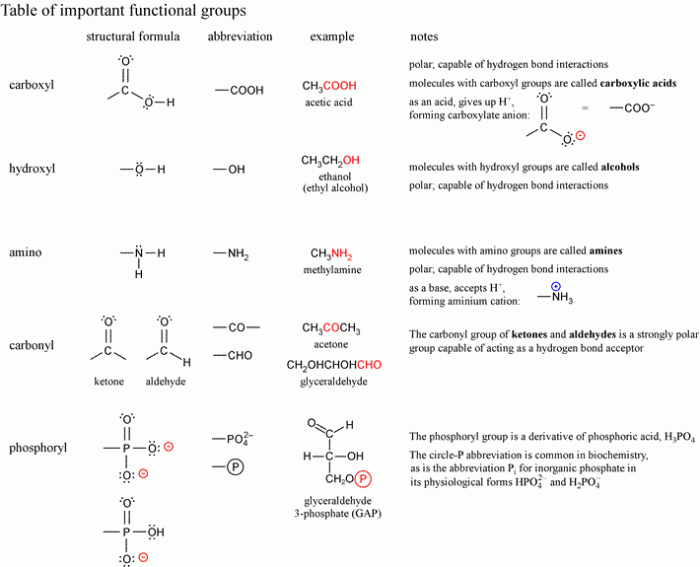
Functional groups determine the reactivity of molecules:
4.1. Nucleophilic Attack
- Nucleophiles (e.g., hydroxyl group) attack electrophilic groups (e.g., carbonyl group)
- Forms new bonds and products
4.2. Oxidation-Reduction Reactions
- Functional groups undergo oxidation or reduction
- Alters the properties and reactivity of molecules
5. Functional Groups in Metabolic Pathways
Functional groups facilitate metabolic reactions:
5.1. Glycolysis
- Hydroxyl groups on glucose undergo phosphorylation
- Carbonyl group of pyruvate undergoes reduction
5.2. Citric Acid Cycle
- Carboxyl groups participate in decarboxylation reactions
- Hydroxyl groups form esters
6. Functional Groups in Drug Design
Functional groups influence drug-target interactions:
6.1. Hydrogen Bonding
- Polar functional groups form hydrogen bonds with target molecules
- Enhances drug binding affinity
6.2. Electrophilic Groups
- Electrophilic groups react with nucleophilic groups on target molecules
- Leads to covalent bond formation and irreversible binding
FAQ Overview
What are functional groups?
Functional groups are specific arrangements of atoms within a molecule that determine its chemical properties and reactivity.
How do functional groups contribute to the structure and function of biological molecules?
Functional groups provide specific chemical properties that allow biological molecules to interact with each other and perform their biological functions.
What is the role of functional groups in drug design?
Functional groups are crucial in drug design as they influence the interactions of drugs with biological targets and determine their pharmacological properties.